Thesis
Earth faces an extinction crisis unlike any in human history. In 2024, extinction rates reached approximately 27K species annually, exceeding the natural background rate of 10-100 species per year. This loss of biodiversity threatens to disrupt ecosystems and food webs that have evolved over millions of years. Conservation efforts now operate against a backdrop where over half of all animal species could disappear by 2050, creating an urgent need for novel approaches to preservation and restoration.
International recognition of this crisis has prompted coordinated global action, with 196 governments adopting the Kunming-Montreal Global Biodiversity Framework in December 2022 at the UN Biodiversity Conference (COP15). Replacing the Aichi Biodiversity Targets, this framework establishes four long-term goals for 2050 and 23 action-oriented targets for 2030, including starting restoration on at least 30% of the planet's degraded lands, coastal areas, and oceans. The agreement commits signatories to reduce annual harmful government subsidies by $500 billion, while mobilizing at least $200 billion per year in domestic and international biodiversity financing.
This policy framework reflects a broader evolution in conservation practice that unfolded in the decades prior to it: a shift from preservation to active restoration. Following the reintroduction of wolves in 1995, the Yellowstone Wolf Project demonstrated how reintroducing apex predators could trigger trophic cascades, regenerating riparian vegetation, and reshaping river morphology. The European Wildlife Bank, established in 2013, pioneered large-scale rewilding across multiple countries, reintroducing native herbivores like European bison and Tauros cattle to restore ecological processes. By 2015, the Pleistocene Park project in Siberia implemented large herbivore reintroduction to restore grassland ecosystems and potentially mitigate permafrost thaw.
Technological breakthroughs have opened possibilities for restoration ecology that were previously confined to science fiction. The completion of the Human Genome Project in 2003 laid the groundwork for advanced genomic research, establishing high-throughput sequencing techniques important for conservation genomics. Platforms such as Illumina’s short-read sequencing, which produces millions of 150-300 base pair fragments, and long-read sequencing, which generates reads exceeding 10K base pairs, enable accurate genome assembly in non-model organisms. Combined with computational algorithms (like FALCON and Canu), these complementary approaches allow researchers to sequence extinct species from degraded museum specimens, where DNA is typically fragmented into short segments, while simultaneously assembling complex repetitive regions using long-read technologies. Early successes in sequencing ancient DNA from specimens like Neanderthals in 2010 and ancient horses in 2013 demonstrated the viability of recovering genetic information from extinct organisms.
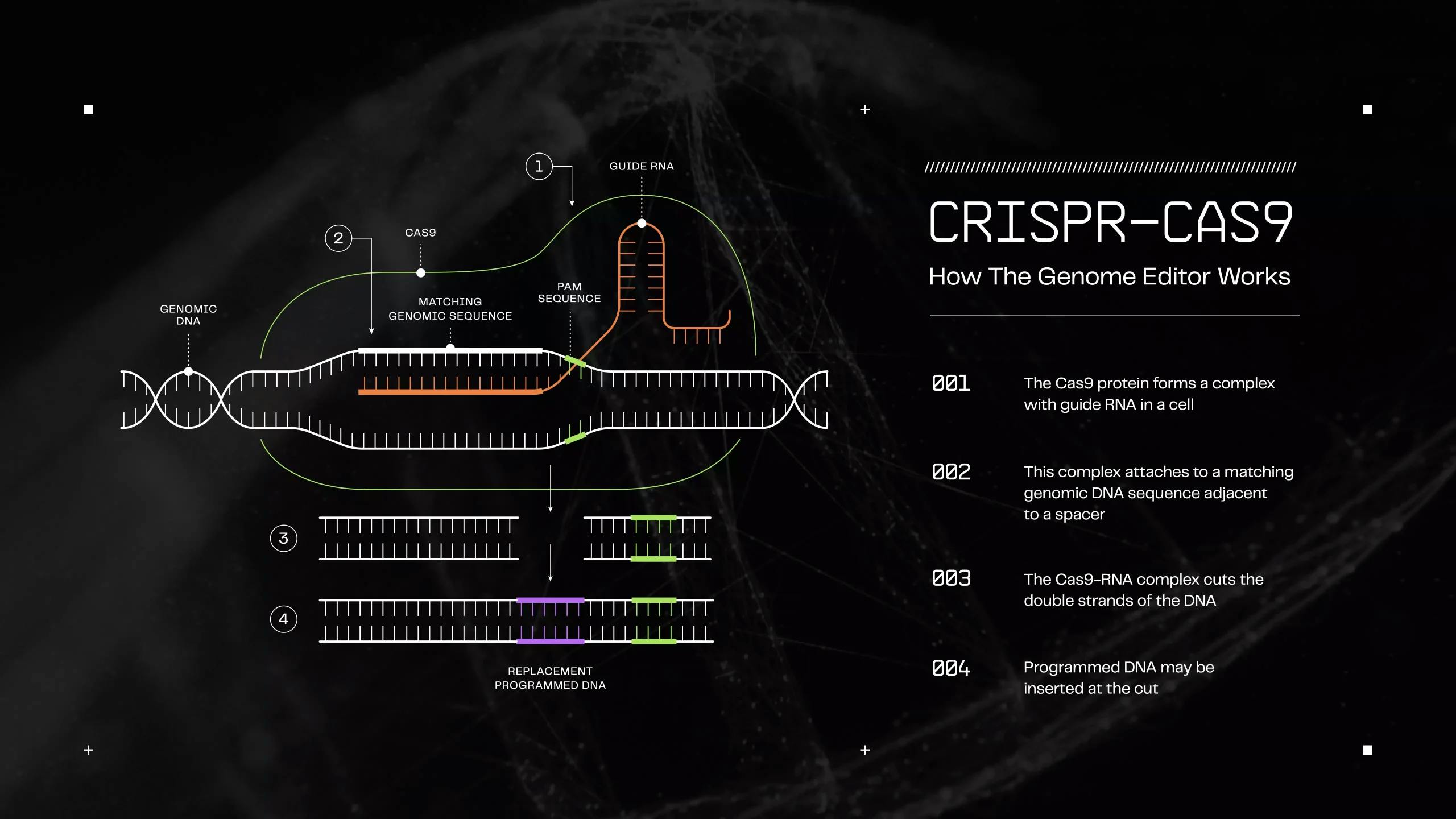
In the early 2010s, CRISPR-Cas9 gene editing technology emerged from research into bacterial adaptive immunity, with researchers discovering that bacteria use CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) and Cas (CRISPR-associated) proteins as a defense mechanism against viral infections. In 2012, Jennifer Doudna and Emmanuelle Charpentier demonstrated that the Cas9 protein could be programmed with synthetic guide RNAs to cut specific DNA sequences at precise locations. The system functions as molecular scissors, creating double-strand breaks at targeted genomic loci, which cells then repair.
In 2015, researchers at the University of Missouri successfully created genetically modified pigs resistant to porcine reproductive and respiratory syndrome virus (PRRSV) by deleting CD163, the cellular receptor for the virus. Optimized guide RNA design algorithms that reduced off-target effects and multiplexed CRISPR assays capable of targeting numerous genomic loci simultaneously set the stage for the simultaneous editing of multiple genes.
Source: Colossal Biosciences
Reproductive technologies have advanced in parallel with genomic and gene editing tools. In the early 2000s, work from the San Diego Zoo developed protocols for gamete cryopreservation in endangered species, specifically for felids and ungulates. By 2012, teams at the Audubon Center successfully transferred nuclei from endangered cats into domestic cat oocytes to create viable embryos. In 2017, researchers at the Children's Hospital of Philadelphia maintained lamb fetuses in ex-utero biobags for four weeks, establishing proof-of-concept for applications in species with high gestational mortality.
These converging technological advancements have created a foundation for both conservation applications and broader commercial opportunities, akin to previous technology transfer successes of NASA. Just as NASA's space exploration efforts yielded everyday inventions like memory foam, scratch-resistant lenses, and insulin pumps, conservation and restoration biotechnology developed for endangered species can find applications in human healthcare, sustainable agriculture, and industrial biotechnology. The genomic tools initially developed for human medicine now enable analysis of endangered species genomes, while reproductive technologies for livestock and human fertility treatments have been adapted for wildlife conservation. This scientific convergence has unlocked dual-use applications across multiple industries, with the synthetic biology market expanding to $12 billion in 2023 and projected to exceed $31.5 billion by 2029.
With a mission to restore Earth to a healthier state through biotechnology, Colossal Biosciences develops wetware, software, and hardware technologies for habitat conservation and species restoration. Specifically, the company advances genomic sequencing, CRISPR gene editing, and reproductive technologies to pursue de-extinction of organisms like the woolly mammoth, thylacine, dodo, and dire wolf. It aims to reintroduce these species back into their former habitats for ecosystem restoration and extend technologies to prevent the extinction of other endangered populations. The company has established partnerships with academic institutions and wildlife organizations globally to advance conservation biotechnology applications. Similar to how NASA’s space exploration led to commercially viable spinoff technologies in various industries, Colossal Biosciences creates commercial spinouts to bring its conservation biotechnologies to genomics and synthetic biology markets beyond restoration efforts.
Founding Story

Source: Colossal Biosciences
Colossal Biosciences was founded in 2021 by Dr. George Church (Lead Genetics Advisor) and Ben Lamm (CEO). Church's formal scientific education began at Duke University, where he completed a bachelor's degree in zoology and chemistry in two years. While at Duke, he worked as a research assistant in X-ray crystallography, combining his interests in computers and biology to study DNA-protein interactions, publishing his first peer-reviewed paper in PNAS (Proceedings of the National Academy of Sciences of the United States of America) in 1977. His doctoral work at Harvard under Nobel laureate Walter Gilbert proved foundational for genomics, developing technology for direct genomic sequencing that would help launch the Human Genome Project in 1984. After brief positions at Biogen Research Corporation and a postdoctoral stint at the University of California, San Francisco, Church joined Harvard Medical School's faculty in 1986.
Over the next three decades, Church established himself as one of the most influential figures in genomics and synthetic biology. As a scientist-entrepreneur, Church founded or co-founded approximately 50 biotechnology companies through his Harvard laboratory. These included Veritas Genetics, which offered whole genome sequencing to consumers; Editas Medicine, which aimed to develop CRISPR drugs for incurable diseases; and Gen9bio, which specialized in synthetic DNA solutions. His research spanned diverse areas of life sciences, including developmental biology, gene and cell therapy, medical genetics, genomics, and bioengineering. In 2006, he launched the Personal Genome Project, creating an open-access resource of human genomic data. Church was part of one of the teams pioneering CRISPR-Cas9 gene editing in 2012, and his lab developed MAGE (Multiplex Automated Genome Engineering) technology that enabled simultaneous editing of multiple genes.
Church's interest in de-extinction emerged publicly in 2013 when he delivered a National Geographic Society talk outlining the possibility of reviving the woolly mammoth, presenting the concept as a series of solvable scientific problems. By 2015, his lab had begun analyzing the woolly mammoth genome and experimenting with modifying elephant cells using mammoth genes. Church’s lab specifically focused on 60 genes that experiments suggested were important in producing distinctive traits of mammoths, including fatty tissue and high-domed skulls. In 2017, his team successfully integrated 45 mammoth genes into the Asian elephant genome, demonstrating that de-extinction had moved from theoretical discussions to practical application.
Also a serial entrepreneur, Lamm began his journey while still a student at Baylor University, where he founded his first company, Simply Interactive, an e-learning software business later acquired by Agile Interactive in 2010. Lamm went on to found and sell Chaotic Moon Studios (a mobile software development company acquired by Accenture in 2015), Team Chaos (a digital gaming company acquired by Zynga in 2016), and Conversable (an AI-driven conversational intelligence platform acquired by LivePerson in 2018). Prior to co-founding Colossal, Lamm established Hypergiant Industries, an enterprise AI company focused on developing solutions for space, defense, and critical infrastructure (later acquired by Trive Capital in 2023).
Following his talk in 2013, Church received $100K in funding from Peter Thiel. However, this amount of funding was not enough to support his research. As a result, Church’s lab often tacked on mammoth research to other, better-funded experiments to continue progressing genetic tools. In 2019, Lamm reached out to Church to arrange a meeting at his laboratory in Boston, intrigued by the press reports about his de-extinction research. Although they first met to talk about the intersection of synthetic biology and AI, Church told Lamm that if he had unlimited capital, he would work to bring back the woolly mammoth to help the Arctic ecosystem and apply the technologies developed to help conservation and human healthcare.
Their initial exploratory discussions evolved into concrete business propositions to support Church’s work, from experiments with DNA to eventually reintroducing a functional woolly mammoth into the wild. After the COVID-19 pandemic, Colossal Biosciences officially launched from stealth in September 2021 with $15 million in seed funding led by Thomas Tull, with participation from investors including Tim Draper, Tony Robbins, and Winklevoss Capital Management. The company, then with 19 full-time employees, established headquarters in Dallas with an office in Austin and research operations in Boston, creating a bicoastal presence that leveraged Church's academic infrastructure.
At the time of its launch, the company’s short-term focus was refining technologies necessary for reintroducing the woolly mammoth into the wild. To help do so, the founding team recruited Dr. Eriona Hysolli from Church's lab as Head of Biological Sciences, bringing expertise in genome editing for the mammoth project. However, at the same time, Lamm identified revenue streams through commercial spinoffs for genetic engineering or reproductive technology, comparing the company’s long-term goals to the research that powered the Apollo space mission and gave rise to GPS and communication satellites.
In its first year after launching, Colossal Biosciences expanded beyond the mammoth to announce the thylacine (Tasmanian tiger) as its second de-extinction candidate in August 2022, partnering with Dr. Andrew Pask at the University of Melbourne. By January 2023, the company launched its third project focused on the dodo, establishing an Avian Genomics Group led by paleogeneticist Dr. Beth Shapiro. As Colossal Biosciences has progressed with new programs, it has continued developing technologies in genome editing, artificial wombs, and cellular reprogramming, positioning the company as much as a technology platform company as a de-extinction venture.
Product
Colossal Biosciences defines de-extinction not as creating perfect genetic replicas of extinct animals, but rather as developing functional proxy species that embody the key traits and ecological roles of their extinct counterparts. This approach to de-extinction relies on a suite of technologies centered around genomic engineering. The process begins with genome sequencing of ancient DNA extracted from preserved specimens of extinct species. Comparative genomic analysis allows the company to identify the genetic basis for key traits that made these extinct species unique from closely related species still alive today.
Colossal Biosciences also uses CRISPR, which allows precise editing of DNA. This genome editing method works by creating genetic scissors that can accurately delete sections of DNA and create cuts where scientists can insert new genes. Rather than creating entirely new genomes, they edit the DNA of the extinct animal's closest living relative to incorporate key genetic traits from the extinct species.
The company uses different reproductive technology approaches depending on the species. Mammals use techniques such as somatic cell nuclear transfer and artificial insemination with surrogate mothers. For birds, where cloning is not feasible, they work with primordial germ cells. The company has also developed artificial womb technology, which would be important for species without suitable living surrogates.
As of November 2025, Colossal Biosciences is focused on five de-extinction projects: woolly mammoth, thylacine, dodo, dire wolf, and moa. The de-extinction technologies have broader applications for conservation, including helping endangered species adapt to changing environments, preserving genetic diversity, and potentially saving critically endangered animals from extinction. The company has already spun off two separate businesses from its developed technology: Form Bio, which provides AI and computational biology platforms for gene therapy, and Breaking, which uses engineered microbes to break down plastic waste.
Woolly Mammoth De-Extinction Project
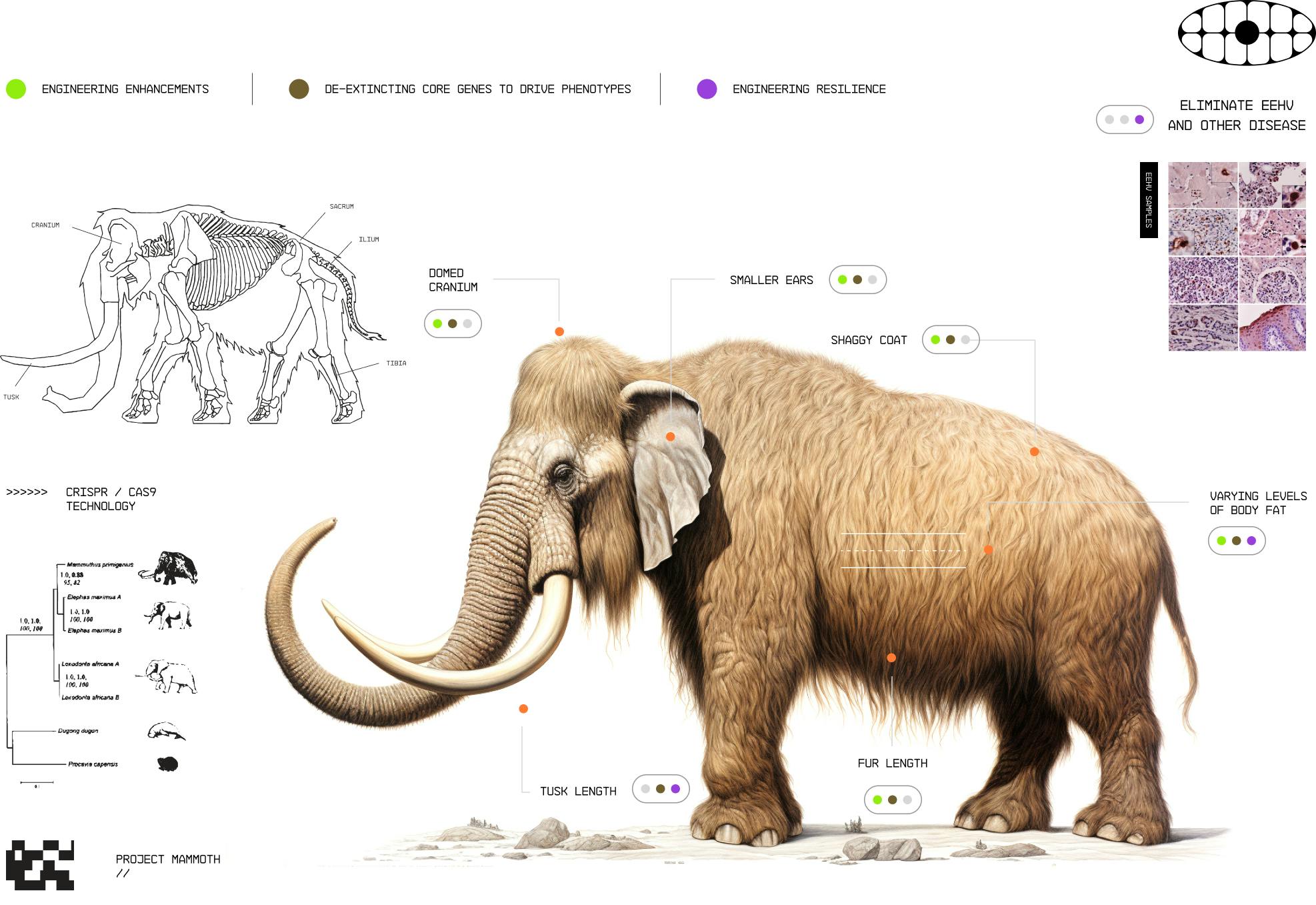
During the company’s launch from stealth in September 2021, Colossal Biosciences announced that the company would pursue the de-extinction of the woolly mammoth as its first program. The stated goals for the program are to increase the resilience of habitats to climate change, understand the genetic basis of cold-adaptation traits for animals, advance multiplex genome editing, and develop tools to contribute to global efforts trying to save modern elephants from extinction.
The woolly mammoth (Mammuthus primigenius) inhabited the steppe-tundras of Eurasia and North America during the Pleistocene epoch between 300K and 10K years ago. When the woolly mammoths still roamed, the tundras were dominated by grasslands, with some researchers arguing that the woolly mammoths were key to preserving this by breaking up moss, knocking trees down, and providing fertilizer through their organic waste. Since then, these tundras once grazed by woolly mammoths across Siberia and North America have seen a 100-fold decrease in total plant and animal biomass compared to when they were steppes, and are now releasing carbon dioxide and rapidly warming. Restoring grasslands could help keep soil from melting and eroding, protecting the Arctic’s permafrost, which stores 1.6 billion metric tons of carbon.
The woolly mammoth’s extinction occurred approximately 4K years ago after millennia of declining population, with the last known woolly mammoths being isolated to islands off the coasts of Siberia and Alaska and possibly killed as a result of a human hunting expedition. The relatively recent deaths of woolly mammoths have provided scientists with well-preserved tissue samples, fur, and tusks in permafrost that contain intact DNA.
Because the woolly mammoth and Asian elephant share 99.6% of their DNA and a common ancestor that lived approximately 6 million years ago, Colossal Biosciences aims to develop a proxy species by incorporating key mammoth genes into the Asian elephant genome. In July 2022, Colossal Biosciences and the Vertebrate Genomes Project (VGP) announced the successful sequencing of the entire Asian elephant genome, marking the first complete sequencing of mammalian genetic code at such a detailed level since the Human Genome Project's completion in the early 2000s. The company has utilized 59 different mammoth genomes that were up to 1.2 million years old to assemble an ancient DNA genome.
Colossal Biosciences combines CRISPR with other DNA-editing enzymes, including integrases, recombinases, and deaminases, to splice woolly mammoth genes into the Asian elephant genome. The mammoth traits they target include a 10-centimeter layer of insulating fat, five different types of shaggy hair, and smaller ears that would help the hybrid tolerate cold weather.
Source: Colossal Biosciences
In March 2024, Colossal Biosciences reported the creation of induced pluripotent stem cells (iPSCs) for Asian elephants. The elephant iPSCs can be multiplex-edited and differentiated to study cold adaptation traits such as woolly hair growth and fat storage in cellular and organoid models. The reprogramming of elephant cells proved challenging due to the complexities of the TP53 pathway in elephants. Their genome contains up to 19 copies of TP53 retrogenes, a core gene that regulates cell growth to prevent cancer, and the reprogramming process for elephants takes longer than for other mammal species.
In March 2025, Colossal Biosciences announced the creation of "woolly mice" with mutations inspired by woolly mammoths. These genetically modified mice exhibit long, wavy, woolly hair of a golden color and accelerated fat metabolism. The woolly mouse experiment involved identifying seven genes that code for the mammoth's shaggy coat (specific genes for coarseness, curliness, and length), one gene for melanin production (giving the golden color), and another governing lipid metabolism. Over five rounds of experiments, scientists produced nearly 250 embryos, with fewer than half developing to larger, more viable 200-300 cell embryos. These were implanted in around a dozen surrogate female mice, resulting in the birth of 38 mouse pups that successfully expressed the desired mammoth traits.

Source: Colossal Biosciences
In March 2025, Colossal Biosciences announced expectations to produce its first woolly mammoth calves by 2028, with mammoth-like Asian elephant embryos produced by 2026 (with a 22-month gestation period required for elephants). In April 2025, the company filed a patent application seeking exclusive rights to create and sell gene-edited elephants containing woolly mammoth DNA. Colossal Biosciences intends to file patents on other transgenic animals, including the de-extincted dire wolves.
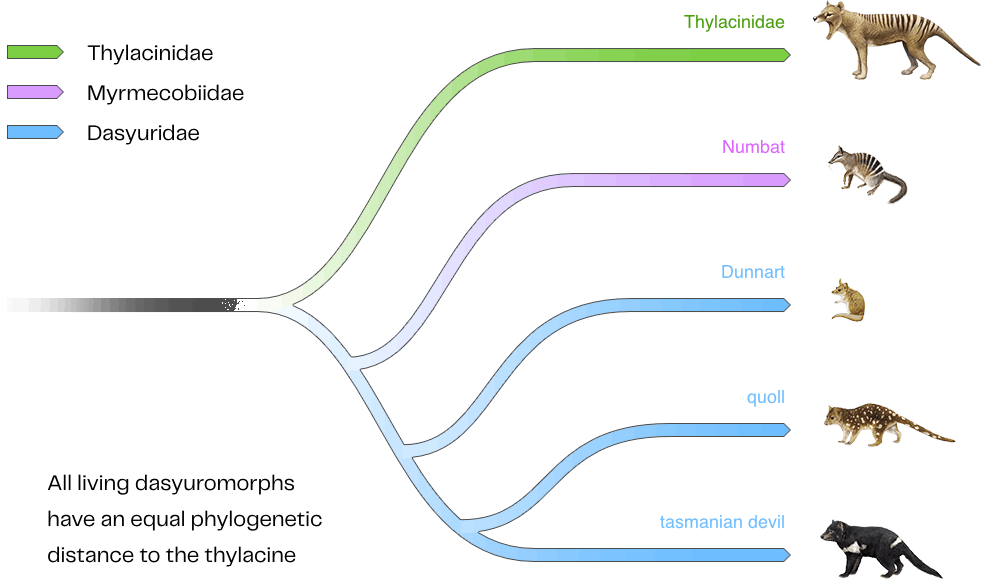
Thylacine De-Extinction Project
The thylacine (Thylacinus cynocephalus), also known as the Tasmanian tiger, was Australia's only marsupial apex predator. Despite its nickname, the thylacine was not related to tigers or other big cats but was a carnivorous marsupial that diverged from other marsupials millions of years ago. Thylacines had distinctive tiger-like stripes across their lower back, a dog-like appearance, and a jaw structure that could open to wide angles.
The species inhabited Tasmania for thousands of years before European colonization. In the 1800s and early 1900s, European settlers blamed thylacines for killing livestock, leading to a government bounty program that resulted in the slaughter of thousands of thylacines. By the mid-1930s, the thylacine population had dwindled to a single individual at the Beaumaris Zoo in Hobart, Tasmania, which died on September 7, 1936, marking the extinction of the species. The thylacine was officially declared extinct in the 1980s.
As an apex predator, the thylacine played a role in maintaining ecosystem balance in Tasmania by hunting non-native predators that preyed on native herbivores. Their extinction created a void in the ecosystem, impacting the control of herbivore populations such as wallabies and kangaroos. Reintroducing the thylacine to selected areas in Tasmania and broader Australia could help rebalance ecosystems that have suffered biodiversity loss since the species disappeared. A restored thylacine population could contribute to controlling overabundant herbivores and selecting sick or weak animals, restoring ecological function to Tasmania's remaining forests.

Source: Colossal Biosciences
In August 2022, Colossal Biosciences announced its partnership with the University of Melbourne's Thylacine Integrated Genomic Restoration Research (TIGRR) Laboratory, led by Dr. Andrew Pask, to launch a thylacine de-extinction research project. Dr. Pask had already been working on thylacine de-extinction for approximately 15 years before the partnership. In 2022, the Wilson Family Trust provided $5 million in philanthropic funding over ten years to establish the TIGRR Lab, and Colossal Biosciences invested an additional $14 million to expand the laboratory's capabilities. In December 2023, Colossal launched the Tasmanian Thylacine Advisory Committee to provide guidance on the project and eventual reintroduction efforts.
The recent extinction of the thylacine has provided scientists with well-preserved specimens in museums and collections. In October 2024, Colossal announced the reconstruction of a thylacine genome claimed to be over 99.9% accurate, with only 45 gaps remaining to be filled through additional sequencing. This achievement was made possible through a preserved 110-year-old thylacine head that had been skinned and preserved in ethanol. The specimen was discovered in a cupboard at Museums Victoria in Melbourne and provided preservation of both DNA and RNA.
The discovery of RNA molecules from the thylacine’s tongue, nasal cavity, brain, and eye tissues was rare, since RNA is typically much less stable than DNA. The RNA data varied between tissues and provided insights into what a thylacine could taste, smell, and see, as well as how its brain functioned. By May 2025, a complete high-quality genome sequencing for the thylacine was achieved.
The fat-tailed dunnart (Sminthopsis crassicaudata), a small mouse-sized marsupial and threatened species, has been identified as a candidate for DNA usage, given its close relation to the thylacine. The de-extinction process will involve using CRISPR gene-editing technology to modify the fat-tailed dunnart genome to more closely resemble that of a thylacine. In October 2024, Colossal announced that it had edited more than 300 unique genetic changes into a fat-tailed dunnart cell line, making it the most edited animal cell to date at the time.
Source: Colossal Biosciences
Colossal Biosciences scientists identified regions of the genome called Thylacine Whole-Animal Regulatory regions (TWARs) that were active in bone tissues of developing thylacines. They tested these regions by replacing sections of mouse genomes with thylacine DNA, finding that these knock-in experiments impacted the development of mouse skulls in predicted ways, confirming that the identified TWARs drive changes in head shape and could give rise to thylacine’s distinctive jaw and skull morphology resembling those of canids.
Following the insertion of a modified cell nucleus into the fat-tailed dunnart egg, Colossal Bioscience will use its assisted reproductive technologies (artificial wombs or in vitro fertilization) to enable embryonic growth and postnatal thylacine joey development. In October 2024, the company announced it had discovered and optimized an approach to induce ovulation in a dunnart, making it possible to control when an animal will come into estrus. Additionally, scientists have been able to take fertilized single-cell embryos and grow them up to halfway through pregnancy using artificial uterus devices. These reproductive technologies have broader applications beyond the thylacine project and could be valuable for conservation efforts for other endangered marsupials, such as koalas and Tasmanian devils.
In October 2024, Colossal Biosciences and the TIGRR Lab reaffirmed their timeline from 2022 of thylacine de-extinction within ten years.
Dodo De-Extinction Project
The dodo (Raphus cucullatus) was a flightless bird endemic to Mauritius that went extinct by 1681, making it one of the earliest and most recognizable symbols of human-caused extinction. Standing around one meter tall and weighing about 15-20 kilograms, the dodo had evolved without fear of predators, which made it particularly vulnerable to human hunters and introduced species that Dutch settlers brought with them.
In January 2023, Colossal Biosciences announced the launch of its Avian Genomics Group to pursue the de-extinction of the dodo as its third species project, following the woolly mammoth and thylacine initiatives. The dodo de-extinction project is led by Dr. Beth Shapiro, who serves as Chief Science Officer to Colossal Biosciences and was the first to fully sequence the dodo's genome. The de-extinction approach involves using the Nicobar pigeon, the dodo's closest living relative and an endangered species, as the foundation for creating a dodo proxy species.
As of January 2025, Colossal Biosciences has generated a complete, high-coverage genome for the dodo and its sister extinct species, the solitaire. The company has also developed a machine learning approach to identify genes associated with craniofacial shape in birds for gene-editing targets toward recreating the dodo's bill morphology. Unlike mammals, birds cannot be cloned using conventional methods and require access to an unfertilized egg cell ready for fertilization. To address the unique challenges of avian reproduction, Colossal Biosciences has translated tools previously developed for chickens for use in dodo de-extinction. The company has processed more than 10K eggs and optimized culture conditions for growing primordial germ cells (PGCs) for four bird species. The PGCs will be used to pair dodo DNA with the genome of the Nicobar pigeon to develop chimeric chicks as surrogates for future dodo restoration. In September 2025, the company announced it successfully grew PGCs in culture for the first time and established a flock of gene-edited chickens that will serve as potential surrogates for dodos and other endangered pigeon species.
In November 2023, Colossal Biosciences announced a partnership with the Mauritian Wildlife Foundation to establish Mauritius as the future home for the de-extinct dodo. The partnership includes plans for applying the technologies developed for the dodo to the conservation of the endangered pink pigeon, of which only about 500 remain in Mauritius. In September 2025, the company created the Mauritius Dodo Advisory Committee (MDAC) to guide conservation strategy and eventual rewilding efforts for the restored dodo.
As of September 2025, Colossal Biosciences expects a timeline of five to seven years for dodo de-extinction.
Dire Wolf De-Extinction Project
The dire wolf (Aenocyon dirus) was a large canid species that roamed North America from approximately 250K to 10K years ago. Larger than modern gray wolves, dire wolves had distinctive features including white coats, more powerful shoulders, wider heads, larger teeth and jaws, more muscular legs, and characteristic vocalizations, especially howling and whining. The dire wolf went extinct during the Late Pleistocene extinction event at the end of the last ice age, but gained cultural recognition through its fictional portrayal in the best-selling novel, A Song of Ice and Fire, and the HBO series TV adaptation, Game of Thrones.
In April 2025, Colossal Biosciences announced the birth of three dire wolf pups, claiming them to be the world's first successfully de-extincted animals. The pups were named Romulus and Remus (males, born October 1, 2024) and Khaleesi (female, born January 30, 2025). All three live in a secure 2K-acre nature preserve at an undisclosed location. The male pups at five months old already weighed approximately 80 pounds and were about five feet long, with an expected weight of 150 pounds when fully grown. They exhibit behaviors that would serve them well in the wild, including howling (which began when they were just two weeks old), stalking, and wolf-like caution, running to hide in dark places when surprised or alarmed.
In October 2025, Romulus and Remus celebrated their first birthday. At 10 months old, the male dire wolves weighed over 115 pounds, already exceeding the typical weight of adult gray wolves in Yellowstone National Park, which average 100-105 pounds. The company announced plans to create additional dire wolves on a faster timeline.

Source: Colossal Biosciences
The de-extinction process involved extracting DNA from ancient dire wolf fossils, including a 13K-year-old tooth found in Ohio, and a 72K-year-old ear bone discovered in Idaho. Creating the dire wolves required making 20 genetic edits in 14 genes of the common gray wolf, which is genetically 99.5% identical to dire wolves. Gray wolf cells were edited to contain the dire wolf version of DNA, creating embryos that were then implanted into surrogate female dogs.
While Colossal Biosciences has claimed this as the first successful de-extinction, some scientists have questioned whether these animals are truly dire wolves or simply genetically modified gray wolves with dire wolf-like characteristics. The scientific debate centers on the definition of de-extinction and whether the functional essence of a species can be restored without complete genetic recreation. In May 2025, Shapiro acknowledged that the de-extincted dire wolves were actually “grey wolves with 20 edits” rather than true dire wolves.
Source: Colossal Biosciences
Alongside the dire wolf project, Colossal Biosciences simultaneously announced the successful cloning of red wolves, a critically endangered species with fewer than two dozen individuals remaining in the wild. The red wolf cloning utilized a novel method of isolating endothelial progenitor cells (EPCs) following a standard blood draw, which is less invasive than traditional cloning techniques that require tissue biopsies. The first cloned red wolf, Neka Kayda, celebrated her first birthday in September 2025.
Moa De-Extinction Project
In July 2025, Colossal Biosciences announced its fifth de-extinction program targeting the South Island giant moa (Dinornis robustus), a large, flightless bird endemic to New Zealand. The moa stood over 10 feet tall and weighed approximately 500 pounds, going extinct around 600 years ago following the arrival of early Māori settlers and subsequent overhunting. As the largest herbivores in New Zealand’s evolutionary history, the moa shaped vegetation structure and nutrient cycling across ecosystems, with the extinction of these herbivores leaving a void across New Zealand’s forests and grasslands. The moa project was announced through a strategic partnership with the Ngāi Tahu Research Centre at the University of Canterbury and filmmaker Sir Peter Jackson, who invested $15 million in the initiative.

Source: Colossal Biosciences
Colossal Biosciences established Colossal Labs New Zealand as a dedicated subsidiary to support the moa project. The company plans to sequence the genomes of all nine historically described moa species. The scientific approach will parallel the dodo project's methodology, using the moa's closest living relatives among ratite birds, like emus, as a genetic foundation for recreating key moa traits through gene editing. In July 2025, the company announced a timeline to de-extinct the moa within five to ten years.
The Colossal Foundation

Source: Colossal Foundation
The Colossal Foundation is a 501(c)(3) organization launched in October 2024 with $50 million in funding focused on the real-world application of Colossal Biosciences-developed science and technology for wildlife conservation and ecosystem restoration. The Colossal Foundation operates as the non-profit arm of Colossal Biosciences, working to implement partner-led conservation initiatives where Colossal Biosciences’ technologies can advance conservation efforts. As part of a ten-year conservation strategic plan developed with conservation partners, the Colossal Foundation funds and deploys new technologies in partnership with local communities.
The Colossal Foundation’s operations can be categorized into three key programs:
Saving Today’s At-Risk Species: The foundation focuses on partnering with conservation efforts to apply genetic rescue to critically endangered species. This involves utilizing Colossal Biosciences’ genetic technologies to support existing conservation programs for species on the brink of extinction. Projects include restoring genetic diversity for the Northern white rhinoceros and Sumatran rhinoceros, rescuing the vaquitas, saving elephants from EEHV (Elephant endotheliotropic herpesviruses), genetically rescuing the pink pigeon, and engineering cane toad toxin resistance in northern quolls.
Vital R&D For Conservation: The foundation partners with other organizations to fund and deploy technologies that leverage artificial intelligence, machine learning, and computational biology to enhance understanding of animal behavior and ecosystems while developing new approaches to preventing extinction. As of November 2025, projects include a drone-based anomaly detection system used by Save the Elephants, an AI-powered bioacoustic monitoring system for locating the tooth-billed pigeon, and an AI-enabled orphaned elephant monitoring system for Elephant Havens in Botswana.
Ensuring Tomorrow’s Biodiversity: The foundation is developing a distributed genetic repository called the Colossal BioVault. This initiative collects tissue samples of endangered species to create accessible cell lines stored in domestic partner facilities. The BioVault serves as an insurance policy against unforeseen threats to biodiversity, focusing on species closest to extinction to preserve their genetic diversity. The foundation is starting with the Colossal 100, which is the top 100 most imperiled species that are targets for this biobanking. Other projects include rediscovering the Victorian grassland earless dragon, which was rediscovered in 2023 after 40 years of disappearance, and searching for over 4.3K other “lost” species.
In August 2025, the Colossal Foundation launched its Species Reintroduction Fund with a $250K annual commitment. The first six projects include reintroducing the Bolson tortoise to New Mexico, restoring the Vietnam phesant, and returning the golden skiffia fish to Mexican waterways. In October 2025, the Colossal Foundation partnered with the BioRescue consortium on a genetic rescue plan for northern white rhinos. The partnership employs a synthetic genetic rescue approach that extracts DNA from historic museum specimens to increase genetic diversity beyond what remains in living and recently deceased individuals. The collaboration projects its first calves within three to four years.
Form Bio

Source: Form Bio
Form Bio is a computational life sciences platform that was launched by Colossal Biosciences in September 2022 as its first technology spinout. The platform serves as a complete solution for managing large datasets, executing verified workflows, visualizing results, and collaborating with peers in the life sciences field. Originally developed to accelerate Colossal's de-extinction projects, Form Bio's technology now addresses broader applications in human health and synthetic biology.
The platform incorporates traditional bioinformatic analysis, machine learning, and an enhanced user interface for gene editing and therapy workflows. It includes genome analysis tools and AI technology that helps identify guide RNAs to assist gene-editing scientists in making predictive cuts with CRISPR technology. Form Bio's system provides visualization tools, validated pipelines for genome assembly and gene-editing design, and creates an online environment for collaboration between researchers. These tools automate repetitive work typically performed by informatics cores at biotech companies, reducing analysis time.
Source: Form Bio
Form Bio specifically addresses limitations faced by cell and gene therapy companies due to patchworks of difficult-to-manage custom code, siloed application and infrastructure components, and antiquated processes. The platform is fully cloud-based, primarily built on Google Cloud Platform but designed to support any cloud environment. For biotech companies, the system enables faster discovery of new therapies, more efficient validation processes, and accelerated movement through regulatory steps. The software was developed to help researchers progress from a scientific idea all the way to a finished medicine or application.
In 2023, Form Bio expanded its offerings with FORMsight AI, an AI-based solution set specifically designed for predicting and optimizing manufacturing outputs of cell and gene therapy constructs. This full-service solution incorporates advanced, customizable AI models, Form Bio's underlying platform, and the expertise of the company's computational life sciences professionals. The FORMsight AI system is available to both cell and gene therapy companies and Contract Development and Manufacturing Organizations (CDMOs). FORMsight AI addresses common issues faced by cell and gene therapy developers, particularly construct truncations and manufacturing contaminations that contribute to low manufacturing yields, delayed times-to-market, and therapeutic safety issues. Using large language models in conjunction with the Form Bio platform, developers can predictively analyze and avoid key manufacturing risk factors before scaling up production.
Breaking

Source: Breaking
Breaking is a plastic degradation company that was launched from Colossal Biosciences in April 2024 as its second technology spinout. The company is developing X-32, a microbial discovery that can degrade multiple types of plastics by breaking down hydrocarbon chains across different plastic types. In its natural state, X-32 can degrade polyolefins, polyesters, and polyamides, leaving behind only carbon dioxide, water, and biomass in as little as 22 months. The technology was developed based on discoveries from the Wyss Institute for Biologically Inspired Engineering at Harvard University.
Breaking's primary focus is addressing the global plastics crisis, which has resulted in 5 billion tons of plastic accumulating in landfills, oceans, and ecosystems, with an additional 390 million tons produced annually. The company has identified numerous potential applications for X-32, including implementation in wastewater treatment facilities, food waste processing, and marine environments where the microbe can be added to microbe-based degradation programs. The company's development team is working on future synthetic genetic edits to make X-32 faster, more efficient, and more effective while ensuring it maintains a harmless environmental impact. Breaking is also investigating the potential to use the biomass generated during the degradation process to produce biofuels, biodegradable plastics, and high-value chemicals, creating a circular approach to plastic waste management. As of April 2024, Breaking hopes to utilize its technology to ensure that newly created plastics have a faster degradation period and smaller overall impact on the environment in the next few years.
Market
Customer
Colossal Biosciences has established several partnerships to advance its research and conservation efforts. In October 2021, Colossal announced its partnership with the Vertebrate Genomes Project (VGP), providing funding for VGP to sequence and assemble Asian, African bush, and African forest elephant genomes for preservation purposes. These genomes were made publicly accessible for research without use restrictions in July 2022 and May 2023, respectively. The company has partnered with zoos, governments, and indigenous peoples across Australia, Mauritius, and Alaska for de-extinction projects. In April 2025, Colossal Biosciences was in advanced talks with North Carolina for the use of conservation tools to rescue the red wolf.

Source: Colossal Foundation
Through the Colossal Foundation, Colossal Biosciences has partnered with 48 organizations as conservation partners to help with the protection and restoration of keystone species. In January 2025, the Colossal Foundation donated $1.5 million to Dr. George Church's lab at the Wyss Institute for Biologically Inspired Engineering at Harvard University to advance artificial womb research for conservation initiatives focused on endangered species. The Colossal Foundation also works with several local partners to empower local communities and Indigenous peoples.

Source: Colossal Foundation
Form Bio targets organizations and professionals in the life sciences sector, particularly those engaged in cell and gene therapy, drug discovery, and computational biology research. Its customers include pharmaceutical companies, biotechnology firms, healthcare organizations, and academic research institutions that require advanced computational platforms to manage large biological datasets, streamline bioinformatics workflows, and accelerate therapeutic development.

Source: Form Bio
As of November 2025, Breaking has not publicly announced any of its customers. The company’s likely target customers include companies and municipal agencies in the waste management, food waste and composting, and wastewater treatment sectors, as well as those operating in marine environments where plastic pollution is a challenge.
Market Size
Colossal Biosciences’ core conservation mission aligns with a global wildlife conservation market valued at approximately $29 billion in 2024 and projected to reach $41.6 billion by 2029 (7.2% CAGR). Emerging conservation financing mechanisms, such as biodiversity credits and habitat banking, are creating new market opportunities for companies with Colossal Biosciences’ technical capabilities. Additionally, the global synthetic biology market was valued at $14.5 billion in 2024 and projected to reach $126.9 billion by 2034, representing a 24.2% CAGR. This expansion stems from technical advancements in DNA synthesis and gene editing, decreasing genetic sequencing costs, and increasing industrial applications across pharmaceuticals, agriculture, and materials science. This market acceleration creates diverse commercialization pathways for Colossal Biosciences’ gene editing and synthetic genomics capabilities beyond de-extinction applications.
Colossal Biosciences’ reliance on advanced gene editing technologies, particularly CRISPR, positions it within a market valued at $9.3 billion in 2024 and projected to reach $40.1 billion by 2034 (15.7% CAGR). The pipeline of gene therapy treatments utilizing these technologies continues to expand, with over 3.9K clinical trials worldwide in 2023 involving gene editing technologies. Colossal Biosciences’ expertise in multiplex gene editing creates commercial opportunities across therapeutic, agricultural, and industrial biotechnology applications.
Through its Form Bio spinoff, Colossal Biosciences has also entered the bioinformatics and computational biology market, which was valued at $14.3 billion in 2024 and forecasted to reach $50.3 billion by 2030 (13.4% CAGR). This growth is driven by increasing biological data volumes requiring sophisticated analysis tools and accelerating adoption of AI and machine learning in life sciences. Form Bio's specialized computational tools create additional revenue streams while supporting Colossal Biosciences’ core research objectives.
Colossal Biosciences has also expanded into plastic-degrading enzymes through its Breaking spinoff, which operates within a market valued at $6.2 billion in 2023 and projected to reach $15.3 billion by 2031 (4.3% CAGR). This market growth is driven by escalating concerns about plastic pollution, increasingly stringent regulations, and corporate commitments to reduce plastic footprints. These environmental applications demonstrate how Colossal Biosciences’ synthetic biology platform can address sustainability challenges beyond de-extinction while creating commercial value.
Competition
Colossal Biosciences focuses on developing gene editing and synthetic biology technologies for its projects across de-extinction, restoration, and conservation. However, the company has demonstrated a strategic approach to commercializing its technologies through spinoff ventures, including Form Bio and Breaking. In April 2025, the company indicated plans to commercialize additional technologies, such as plans for its advancements in artificial wombs and reproductive biology.
The core technologies Colossal Biosciences has developed for de-extinction projects, including advanced gene editing, computational genomics, and reproductive technologies, have broad commercial applications across multiple industries. These technologies were initially developed to solve the challenges of restoring extinct species, but can be repurposed to address challenges in agriculture, medicine, materials, and environmental remediation. While Colossal Biosciences does not face substantial competition for its de-extinction programs, its current and future commercial spinoffs used to generate revenue streams face competitors across gene editing and synthetic biology.
The competitive landscape in synthetic biology reflects the diverse commercial opportunities available to Colossal Biosciences. In agricultural biotechnology, companies like Inari, Pairwise, and Tropic Biosciences are leveraging gene editing to develop improved crop varieties, while Roslin Technologies and Genus focus on genetic improvement of livestock or cultivated meat production. In medicine, Ginkgo Bioworks engineers custom microorganisms for various applications, eGenesis develops xenotransplantation solutions, and Fauna Bio uses animal genomics to identify novel therapeutics. Colossal Biosciences’ background in genomic engineering places it in a position to enter any of these markets through strategic spinoffs, partnerships, or licensing arrangements.
The next-generation gene editing landscape offers another avenue for Colossal Biosciences’ commercial expansion. Companies including Tessera Therapeutics, Metagenomi, Arbor Biotechnologies, Mammoth Biosciences, Inscripta, and Beam Therapeutics are developing specialized gene editing tools and platforms with applications ranging from human therapeutics to industrial biotechnology. Colossal Biosciences' multiplex gene editing capabilities, refined through its de-extinction work, could be commercialized for applications requiring complex, precise genomic modifications.
Finally, Colossal Biosciences’ spinoffs, Form Bio and Breaking, also face competition. Form Bio operates in the bioinformatics and computational biology market, competing with established players like Benchling and DNAnexus that offer end-to-end laboratory management systems, as well as newer entrants like LatchBio with its cloud-based collaborative platform. Meanwhile, Breaking's focus on enzymatic plastic degradation places it in competition with companies like Samsara Eco, which has commercial enzyme technologies for plastic recycling, and Novoloop, which uses chemical processes to transform plastic waste into new materials.
Synthetic Biology for Agriculture
Inari: Founded in 2016 as a Flagship Pioneering portfolio company, Inari is an agricultural biotechnology company developing gene-edited seeds using its computational and multiplex gene editing platform called SEEDesign. In January 2025, the company secured $144 million in Series G funding. As of November 2025, Inari has raised $753 million in total funding from investors such as G Squared, Alexandria Venture Investments, and the Abu Dhabi Investment Authority.
Inari’s technology combines AI prediction models, gene editing, and multiplexed engineering to develop crop varieties with improved yield, sustainability, and climate resistance. The company has established partnerships with seed companies and is working on staple crops, including corn, soybeans, and wheat. Inari targets specific genetic pathways that control complex traits while accelerating the breeding timeline from over 10 years to 3-5 years. Although Colossal Biosciences focuses on de-extinction of animals, commercial spinoffs using its multiplex gene editing technology could be focused on agricultural biotechnology to improve crops or habitat resilience, overlapping with Inari’s existing products and services.
Pairwise: Pairwise is an agricultural biotechnology company founded in 2017 using CRISPR gene editing to develop improved fruit and vegetable varieties. As of November 2025, the company had raised $155 million in total funding, including a $40 million Series C round in September 2024 led by Deerfield Management. Other investors include Monsanto Growth Ventures, Leaps by Bayer, and Aliment Capital. Pairwise's proprietary CRISPR-based gene editing platform enables precise modifications to plant genomes.
In March 2023, the company launched its first commercial product, Conscious Greens, featuring gene-edited mustard greens with reduced bitterness. Pairwise has strategic partnerships with Bayer Crop Science to improve row crops and with the USDA Agricultural Research Service to accelerate breeding in berries and other specialty crops. Similar to Colossal Biosciences, the company's editing capabilities can introduce multiple genetic changes simultaneously, and its core technology would overlap with Colossal Biosciences if it began applying its synthetic biology solutions to plants or agriculture.
Tropic Biosciences: Founded in 2016, Tropic Biosciences specializes in applying advanced gene editing technologies to tropical crops like bananas, coffee, and rice. As of November 2025, the company had secured over $73.5 million in total funding, including a $35 million Series C round in July 2022 led by Blue Horizon Corporation. The proprietary GEiGS (Gene Editing Induced Gene Silencing) technology of Tropic Biosciences allows for precise modification of crop traits. The company has developed disease-resistant banana varieties to combat Panama disease, which threatens global banana production, and reduced-caffeine coffee plants that naturally produce less caffeine during growth. As with Colossal Biosciences and its approach in animal genomes, Tropic Biosciences applies CRISPR gene editing to solve agricultural challenges, though in tropical crops rather than extinct species.
Roslin Technologies: Founded in 2017 as a commercial arm of the Roslin Institute, Roslin Technologies develops animal cell technologies and genetic engineering solutions for agriculture and food production. The company secured £11 million in Series A funding in November 2022, led by Novo Holdings, bringing total investment to over $27.8 million as of November 2025. Roslin Technologies has developed pluripotent animal stem cell lines for cultivated meat production and genetic engineering applications, with a library covering pigs, cows, sheep, and salmon. Roslin's focus on animal cell technology and genetic engineering parallels the work of Colossal Biosciences, though with applications in food production and livestock improvement rather than de-extinction.
Genus: Genus is an animal genetic improvement company founded in 1933. It is publicly traded on the London Stock Exchange and, as of November 2025, had a market cap of $2.1 billion. Genus operates through two main divisions: PIC (Pig Improvement Company), which provides genetically superior breeding stock to pork producers, and ABS Global, which offers bovine genetics and reproductive services. In collaboration with the University of Missouri, Genus has developed PRRS-resistant pigs using gene editing technology to combat a virus that costs the pork industry billions annually. Unlike the focus on extinct species for Colossal Biosciences, Genus applies genomic technologies to commercially relevant livestock, though both companies utilize genetic engineering to modify animal genomes.
Synthetic Biology for Medicine
Ginkgo Bioworks: Founded in 2008, Ginkgo Bioworks operates a synthetic biology platform that designs and engineers custom microorganisms. The company went public through a $1.6 billion SPAC in September 2021 and trades on the NYSE. In November 2025, the company had a market cap of ~$700 million. Ginkgo Bioworks’ platform combines automation, software, and biological expertise to engineer organisms for applications across multiple industries, including pharmaceuticals, agriculture, food, materials, and enzymes.
The company operates on a business model where it develops organisms for customers and earns revenue through upfront fees, milestone payments, and royalties on successful commercialization. Both Colossal Biosciences and Ginkgo Bioworks focus on utilizing synthetic biology platforms to manipulate genetic material, and future commercial spin-offs from Colossal Biosciences could overlap with Ginkgo Bioworks’ services if they concern applications for biopharma and enzymes for chemicals or materials.
eGenesis: eGenesis was co-founded in 2014 by George Church (who also co-founded Colossal Biosciences) and focuses on using advanced gene editing to develop humanized organs for transplantation. The company had raised $456 million in total funding as of November 2025, including a $191 million Series D round in September 2024 led by Lux Capital. Other investors include ARCH Venture Partners, Khosla Ventures, and Farallon Capital Management.
eGenesis' multiplex gene editing platform enables the company to make many genetic modifications simultaneously to pig organs, addressing barriers to xenotransplantation, including viral risk, immune compatibility, and physiological incompatibility. In April 2025, the FDA cleared clinical trials to test whether eGenesis’ livers from gene-edited pigs could treat people with sudden liver failure. Both Colossal Biosciences and eGenesis apply genome engineering to animals, although eGenesis develops transplantable organs and Colossal Biosciences has limited activity in reproductive technologies and artificial wombs.
Fauna Bio: Founded in 2018, Fauna Bio leverages genomic data from animals to develop novel human therapeutics. In December 2023, the company announced a multi-year partnership with Eli Lilly worth up to $494 million to support AI discovery initiatives in obesity. Fauna Bio's Convergence platform combines multi-species genomic analysis with AI to identify protective genetic mechanisms in hibernating mammals that could be translated into human therapies. The company uses transcriptomic, epigenomic, and proteomic data across 290+ animals and 20+ time points. Colossal Biosciences' work with ancient animal genomes and biobank development could be used for therapeutics applications similar to Fauna Bio’s leveraging of animal genomics.
Next-Generation CRISPR & Gene Editing Tools
Tessera Therapeutics: Founded in 2018 as a Flagship Pioneering company, Tessera Therapeutics is a genetic medicines company pioneering Gene Writing technology to cure diseases at their genetic source. As of November 2025, the company had raised $623.1 million in total funding, including a $300 million Series C round in April 2022. Other investors include T. Rowe Price, Alaska Permanent Fund, SoftBank Vision Fund 2, and Qatar Investment Authority.
Tessera Therapeutics' Gene Writing platform allows for precise insertion, deletion, or modification of DNA sequences, enabling both small and large genetic changes without requiring double-stranded DNA breaks. The company's technology enables lasting genetic cures through writing new DNA into the genome. Similar to Colossal Biosciences, Tessera Therapeutics uses genetic engineering technologies, though Tessera Therapeutics focuses on leveraging proprietary gene editing tools for human therapeutic applications.
Arbor Biotechnologies: Founded in 2016, Arbor Biotechnologies develops next-generation gene editing technologies for therapeutic applications. In March 2025, the company raised a $73.9 million Series C round led by ARCH Venture Partners and TCG Crossover. As of November 2025, the company had raised $304.5 million in total funding from investors, including T. Rowe Price, Illumina Ventures, Vertex Pharmaceuticals, and Qatar Investment Authority.
Arbor Biotechnologies’ discovery platform combines machine learning, genome sequencing, and high-throughput screening to identify novel CRISPR nuclease enzymes with properties beyond traditional Cas9. The company's proprietary CRISPR-Cas13d system enables precise RNA editing, while its Cas12i nuclease provides improved specificity for DNA editing applications. Arbor Biotechnologies’ focus on therapeutic applications of its nucleases differs from Colossal Biosciences’ restoration focus, although Colossal Biosciences could expand into biopharma applications of its developed CRISPR gene editing technology.
Mammoth Biosciences: Mammoth Biosciences was founded in 2017 and develops CRISPR applications for disease detection and therapeutics. As of November 2025, the company had raised $359.5 million in total funding, including a $150 million Series D round in September 2021 led by Redmile Group. Other investors include Foresite Capital, 8VC, Amazon, and Sixth Street.
Mammoth Biosciences' CRISPR platform has discovered and characterized novel Cas proteins, including ultra-compact Cas14 and Cas12a variants with enhanced specificity and versatility. The company develops its CRISPR-based technologies for applications in therapeutics, diagnostics, and bio-manufacturing. Although Mammoth Biosciences’ development of novel CRISPR nucleases as its core business differs from Colossal Biosciences’ de-extinction projects, Colossal Biosciences could expand into therapeutics development, like Mammoth Biosciences, in future years to commercialize its gene editing tools and technology.
Inscripta: Founded in 2015, Inscripta develops gene editing technologies and instruments for genome engineering across agriculture, chemicals, and pharmaceuticals. As of November 2025, the company had raised $463.9 million in total funding, including a $150 million Series E round in April 2021 led by Fidelity and T. Rowe Price. Other investors include Venrock, Paladin Capital Group, and Foresite Capital.
The company's MAD7 CRISPR nuclease provides an alternative to Cas9 with distinct targeting properties. Inscripta's technology enables researchers to make tens of thousands of edits simultaneously across a genome, accelerating strain development for industrial biotechnology applications. The company's focus on enabling large-scale genomic modifications through automated systems differs from Colossal Biosciences' de-extinction mission, though both leverage advanced gene editing capabilities, and Colossal Biosciences could spin off its multiplex gene editing technologies for use by other companies across industries.
Beam Therapeutics: Beam Therapeutics was founded in 2017 to commercialize base editing technology. The company went public through a ~$207 million IPO in February 2020 and, as of November 2025, had a market cap of $2.4 billion. Beam Therapeutics’ proprietary base editing technology enables precise conversion of single DNA bases without inducing double-strand breaks, allowing for the correction of point mutations that cause genetic diseases.
The company employs multiple delivery systems, including lipid nanoparticles and adeno-associated viruses, to target edits to specific tissues. Beam Therapeutics' pipeline includes candidates for sickle cell disease and alpha-1 antitrypsin deficiency (AATD), with its lead candidate BEAM-101 for sickle cell disease in Phase 1/2 clinical trials, as of November 2025. Although Beam concentrates on human therapeutics, both it and Colossal Biosciences utilize genetic engineering technologies that enable precise DNA modifications.
Bioinformatics & Gene Therapy Software
Benchling: Founded in 2012, Benchling provides a cloud-based platform for life sciences research and development. As of November 2025, the company had raised over $411.9 million in total funding, including a $100 million Series F round in November 2021 led by Altimeter Capital and Franklin Templeton. Other investors include Sequoia Capital, Thrive Capital, Benchmark, Andreessen Horowitz, and Menlo Ventures.
Benchling's platform integrates laboratory information management, electronic lab notebooks (ELNs), molecular biology tools, and data analytics in a unified, collaborative environment. The platform supports workflows across drug discovery, enzyme engineering, agricultural biotechnology, and materials science, and enables researchers to design, execute, and analyze experiments while maintaining full data lineage and compliance with regulatory requirements. While Benchling offers solutions across laboratory operations, Form Bio focuses only on providing tools to accelerate gene therapy development. Both Form Bio and Benchling offer computational and design tools for gene therapies, although Form Bio also offers AI-powered in-silico assays to explore more candidates.
DNAnexus: DNAnexus was founded in 2009 and provides a cloud-based platform for genomic and biomedical data analysis. As of November 2025, the company had secured approximately $472.6 million in total funding, including a $200 million funding round in March 2022 led by Blackstone Growth. Additional investors include Google Ventures, Foresite Capital, and Northpond Ventures. DNAnexus's platform combines cloud infrastructure with bioinformatics tools to enable secure storage, analysis, and sharing of genomic data at scale. DNAnexus’s specialization in large-scale genomic data management across clinical and research environments differs from Form Bio’s focus on gene therapy computational and design tools.
LatchBio: Founded in 2021, LatchBio provides a cloud-based collaborative platform specifically designed for computational biology workflows. As of November 2025, the company had raised approximately $33.2 million in total funding, including a $28 million Series A round in August 2022 led by Lux Capital. Other investors include General Catalyst, Caffeinated Capital, and Fifty Years. LatchBio's platform enables researchers to create, share, and execute computational workflows for genomics, proteomics, and other biological data types without requiring programming expertise. The company also offers data infrastructure for engineering gene editing proteins and tools for analyzing genome-scale screening data. Although both Form Bio and LatchBio offer computational tools for gene therapy and editing technologies, Form Bio also offers in-silico assays to improve candidate exploration.
Plastic Degradation & Recycling
Samsara Eco: Founded in 2021, Samsara Eco is developing enzymatic recycling technology to break down plastics into their original chemical building blocks. In June 2024, the company raised a $65 million Series A round, bringing its total funding to $107 million as of November 2025.
Samsara Eco uses engineered enzymes to depolymerize various plastic types, including PET, polyester, and nylon, into their constituent monomers, which can then be used to create new, virgin-grade plastics. The company's process operates at ambient temperature and pressure, requiring less energy than traditional mechanical or chemical recycling methods. Similar to Samsara Eco, Breaking also focuses on engineering enzymes for plastic degradation, although it is in the process of optimizing microbe-produced enzymes and does not yet have commercial applications, as of November 2025.
Novoloop: Founded in 2015 as BioCellection before rebranding to Novoloop, the company develops advanced chemical recycling technology for plastic waste. As of November 2025, Novoloop had raised $45.1 million in total funding, including a $21 million Series A in June 2022. Novoloop transforms polyethylene waste, including difficult-to-recycle plastic bags and packaging films, into performance materials and specialty chemicals. The company's chemical alteration of post-consumer polyethylene produces a thermoplastic polyurethane that offers performance comparable to virgin materials for applications in footwear, sporting goods, and automotive parts. While Breaking focuses on degrading plastics using enzymes, Novoloop transforms plastic waste into high-performance materials through chemical processes.
Business Model
Colossal Biosciences operates on a multi-faceted commercial approach and plans to generate revenue through multiple streams, including technology spinouts, government contracts for conservation services, and the potential sale of biodiversity credits. Biodiversity credits are a market-based mechanism similar to carbon credits that would become viable if the company successfully reintroduces extinct species into their respective ecosystems. Colossal Biosciences’ CEO has suggested that each woolly mammoth that is brought back could generate up to $2 million in carbon capture services from the resulting ecosystem and climate restoration.
The company's monetization strategy largely stems from technologies developed during its de-extinction research efforts rather than from the revived species themselves. As of November 2025, Colossal Biosciences has already spun out two businesses: Form Bio and Breaking. The company plans to spin off three additional businesses over the next two years, one of which will focus on artificial womb technology with potential applications in fertility treatment.
As of November 2025, Colossal Biosciences offers its conservation technology to governments at no cost. However, government collaborations represent another potential revenue stream for preserving species or de-extinction of culturally or spiritually significant animals. Ecotourism could also be a potential revenue source in partnership with governments and indigenous people, where visitors could see reintroduced species.
Colossal Biosciences maintains a workforce of over 170 scientists and 95 advisors distributed across four specialized laboratories (including the Church Lab, TIGRR Lab, and two other labs in Boston and Dallas) as of January 2025. These teams are divided by project and span disciplines including computational biology, cell and genome engineering, stem cell biology, embryology, protein engineering, and assisted reproductive technologies. Colossal Biosciences also maintains partner laboratories across the world.

Source: Colossal Biosciences
Traction
The company has secured early government partnerships, and as of January 2024, the only revenue it has is through this federal spending. The company has achieved scientific milestones that demonstrate progress toward its goals, including the previously mentioned birth of "woolly mice" with mammoth-like fur and the birth of dire wolves. As of April 2024, Colossal Biosciences’s first commercial spinoff, Form Bio, has established market presence, generating seven figures in ARR.
Valuation
In January 2025, Colossal Biosciences raised a $200 million Series C led by TWG Global at a $10.2 billion valuation. In September 2025, the company raised an additional $120 million as an extension of its Series C round, bringing total Series C funding to $320 million. USIT led this extension, with participation from Peter Jackson, who committed $15 million specifically to the giant moa de-extinction project and partnership with Ngāi Tahu Research Centre. This followed its $150 million Series B funding raised two years prior, which was led by USIT (United States Innovative Technology Fund). As of November 2025, Colossal Biosciences had raised $568.1 million in total funding from investors including IQT, JAZZ Venture Partners, and Breyer Capital.
In September 2022, Form Bio, the first technology spinout from Colossal Biosciences offering a computational life sciences software platform, raised $30 million in Series A funding led by JAZZ Venture Partners. Thomas Tull, one of the lead investors in the $60 million Series A round for Colossal Biosciences in March 2022, also participated. In April 2024, Breaking, the second technology spinout from Colossal Biosciences targeting plastic degradation, emerged from stealth with $10.5 million in seed funding.
Key Opportunities
Accelerating Biodiversity Crises
The acceleration of species loss creates market demand for conservation solutions that Colossal Biosciences is positioned to address. In 2015, it was estimated that extinction rates stand at 100-1K times higher than natural background levels, with the United Nations reporting in 2019 that one million species were at risk of extinction in the coming decades. The escalating crisis has shifted from a peripheral environmental concern to a central economic and ecological challenge requiring new technological approaches. As ecosystem collapse risks become evident across multiple biomes, traditional conservation methods alone may prove insufficient, creating openings for Colossal Biosciences’ synthetic biology-driven interventions that can complement and extend conventional conservation efforts.
Growing scientific recognition of permafrost thaw risks creates specific opportunities for Colossal Biosciences’ mammoth resurrection project. The increasing focus on Arctic ecosystem restoration as a climate stabilization strategy aligns with Colossal Biosciences’ vision for mammoth-elephant hybrids as ecosystem engineers. The potential for Arctic landscape management through controlled grazing to maintain grasslands instead of carbon-releasing forests presents an application of de-extinction technology with climate mitigation benefits.
Expansion of Synthetic Biology
The global synthetic biology market was valued at $14.5 billion in 2024 and projected to reach $126.9 billion by 2034, representing a 24.2% CAGR. This expansion stems from technical advancements in DNA synthesis and gene editing, decreasing genetic sequencing costs, and increasing industrial applications across pharmaceuticals, agriculture, and materials science. This market acceleration creates diverse commercialization pathways for Colossal Biosciences’ gene editing and synthetic genomics capabilities beyond de-extinction applications.
The synthetic biology sector presents commercialization opportunities for Colossal Biosciences beyond de-extinction. With the market projected to grow at annual rates exceeding 20%, opportunities span pharmaceuticals, agriculture, and materials science, which all provide multiple commercialization pathways for gene editing and synthetic genomics technologies. Declining gene sequencing and synthesis costs are accelerating adoption across industries, lowering barriers to market entry for companies with advanced genomic capabilities. This could create diverse revenue streams that can support Colossal Biosciences’ conservation mission while establishing commercial sustainability.
Colossal Biosciences’ genomic editing platforms developed for de-extinction translate to disease model development and pharmaceutical testing, while expertise in genetic diversity and genome manipulation is directly relevant to personalized medicine and rare disease therapies. Advanced reproductive technologies, such as those designed for surrogate species, have potential uses in fertility treatments and regenerative medicine, representing high-value commercial opportunities with clearer regulatory pathways.
The agricultural sector offers further commercialization potential for Colossal Biosciences’ genetic engineering and reproductive technologies. Techniques developed for genetic rescue and trait reactivation in extinct species are applicable to genetic preservation and enhancement of agricultural biodiversity, crop resilience, and climate adaptation. Advanced reproductive technologies can also be used for livestock breeding optimization and genetic resource preservation, aligning with demand for sustainable food systems and climate-adaptive crops.
Colossal Biosciences’ genomic and DNA recovery technologies have applications in environmental monitoring and restoration. Techniques developed for ancient DNA analysis can be applied to environmental DNA (eDNA) monitoring, a tool for biodiversity assessment and ecosystem management. Synthetic biology approaches enable the design of engineered organisms for bioremediation and pollution management, with engineered microbes showing promise in breaking down pollutants and restoring ecological balance.
Regulatory & Financing Frameworks
The evolution of biodiversity credit standards creates an opportunity for Colossal Biosciences to help shape and benefit from emerging ecological asset markets. Multiple international initiatives are actively establishing biodiversity metrics and valuation methodologies that could incorporate de-extinction and genetic rescue projects. The emerging consensus on biodiversity credit frameworks provides Colossal Biosciences with potential monetization pathways for its conservation outcomes, creating financial sustainability beyond venture funding. These standardization efforts establish the infrastructure necessary for Colossal Biosciences to potentially position de-extinction projects as biodiversity assets with quantifiable ecosystem service benefits.
The strengthening of biodiversity components within corporate ESG frameworks creates potential partnership and funding opportunities for Colossal Biosciences' technologies. Increasing biodiversity reporting requirements across jurisdictions drives corporations to seek measurable biodiversity improvements within their operational footprints. Supply chain transparency initiatives further amplify awareness of ecosystem impacts throughout global production networks, creating demand for new restoration approaches. Growing shareholder activism around biodiversity risks places additional pressure on corporations to demonstrate meaningful biodiversity actions beyond traditional offsets. These trends create potential corporate partners for Colossal Biosciences as companies seek differentiated, high-impact biodiversity initiatives that align with their sustainability narratives and compliance requirements.
Key Risks
Revenue & Valuation Skepticism
As of November 2025, Colossal Biosciences’ revenue generation remains largely speculative, with its only revenue as of January 2024 being from some federal deals and several million in ARR for Form Bio. This lack of revenue has led some to question its valuation of $10.2 billion in January 2025, especially since financing for Colossal Biosciences and its spinoffs appears to rely on repeat investors.
Notable repeat investor Thomas Tull, who led the seed funding round and co-led the Series A round, is the founder and chairman of USIT (the lead investor for Colossal Biosciences’ $150 million Series B in January 2023) and jointly leads TWG Global as co-chairman (the sole financier of Colossal Biosciences’ $200 million Series C that valued the company at $10.2 billion in January 2025). TWG Global was formed as a joint venture that rolled up several of Tull’s firms, including both arms of USIT. Form Bio’s investors across its seed and Series A rounds include only one non-Colossal Biosciences investor, Salem Partners, while all other investors also participated in past Colossal Biosciences funding rounds, including Jazz Venture Partners, Liquid 2 Ventures, Kitty Hawk, and Thomas Tull.
Breaking’s investors across its seed funding round also include only one non-Colossal Biosciences investor, Carnite Ventures, while all other investors also participated in past Colossal Biosciences funding rounds, including Animal Capital, Climate Capital, and Builders VC. While this could raise questions about broader market confidence, public opinions could change over time as the company continues to showcase its scientific developments and potentially revenue-generating technologies and spin-offs.
Technical Feasibility
Critics of Colossal Biosciences’ de-extinction claims point out that its resulting animals will be hybrids rather than identical copies of extinct species. Scientists can only approximate extinct species' genomes, creating modified versions of existing animals rather than perfect replicas. This limitation extends beyond technical constraints into philosophical territory, where scholars debate whether genetically modified hybrids truly constitute the resurrection of extinct species. Although Colossal Biosciences has laid out its definition of de-extinction, it must overcome hurdles in public opinion to clarify how it views the de-extinction process and its developed animals.
Additionally, the path from edited embryos to viable offspring encounters obstacles across different taxonomic groups. Colossal Biosciences’ woolly mammoth experiments show that less than half of the engineered embryos reached viable stages, highlighting the low success rates inherent in these processes. Surrogate limitations further complicate species revival, as suitable host animals must be genetically compatible enough to carry engineered embryos while potentially being much smaller than the original species. Artificial womb development remains a solution, but faces uncertain development timelines and unproven scalability for larger species like mammoths.
Reintroducing extinct species into modern ecosystems also carries risks of unintended ecological consequences. Some scientists raise concerns about the potential disruption of existing ecological balances and the competitive displacement of current species occupying similar niches. Reintroduced species could become invasive in modern ecosystems, particularly given the absence of natural predators or controlling factors that existed in their original time periods. Even successfully engineered species may develop unpredictable behavioral adaptations to modern environments that differ from their ancestors. Unknown disease vectors or microbial interactions could also emerge, as extinct species may carry or be susceptible to pathogens that interact unpredictably with modern organisms. These ecological uncertainties extend the timeline for safe species reintroduction and increase regulatory scrutiny.
Ethical & Social Acceptance
Fears of a Jurassic Park-like scenario permeate public discourse around de-extinction, reflecting deeper anxieties about unintended consequences and the responsible governance of powerful biotechnologies. Colossal Biosciences’ CEO Ben Lamm has acknowledged the potentially dangerous nature of the core technologies involved, including CRISPR, AI, and synthetic biology. Religious and philosophical opposition to what some characterize as "playing god" creates additional resistance among certain segments of society, potentially limiting social license for de-extinction projects.
Colossal Biosciences also faces scrutiny over resource prioritization as critics question the allocation of substantial financial and scientific resources toward de-extinction rather than addressing immediate conservation crises. There are concerns within conservation communities that de-extinction projects may divert attention and funding from critically endangered living species that could be saved through conventional conservation approaches.
Ethical questions also surround the welfare implications for engineered animals and surrogate mothers involved in de-extinction processes. Some have raised concerns about potential suffering during experimental procedures, with particular focus on surrogate mothers who may experience complications during pregnancy with genetically modified embryos. The potential for developmental abnormalities or chronic health issues in de-extinct species raises additional welfare concerns, as these animals may face physiological challenges not anticipated in the engineering process.
The broader moral debate centers around whether creating animals for ecosystem services (or, in the view of some, to satisfy human curiosity or for technological achievement) is ethically justifiable based on human responsibilities toward engineered organisms and potential dangers in ecological disruption or other future applications of the technology.
Uncertain Regulatory Outlook
Colossal Biosciences faces regulatory ambiguity as de-extinction technologies operate in uncharted legal territory. The legal classification of de-extinct species remains undefined, creating uncertainty whether they qualify as endangered, experimental organisms, or GMOs, with each classification carrying different regulatory requirements and ownership implications that affect commercialization pathways. Patent questions surrounding genetically engineered organisms add another layer of complexity, with unclear precedents for securing intellectual property rights over recreated species.
Additionally, cross-border species reintroduction plans encounter inconsistent regulatory landscapes, with each potential habitat country maintaining different conservation and biosafety regulations. This regulatory fragmentation creates project risks as approval in one jurisdiction provides no guarantee of acceptance elsewhere. The timeline for obtaining release permissions introduces delays through required environmental impact assessments, stakeholder consultations, and multi-jurisdictional approvals, each with different standards and timelines. As de-extinction technologies advance, regulatory bodies worldwide are developing new oversight frameworks that could impose restrictive conditions, extensive testing requirements, or even moratoriums in some regions. Political opposition to genetic engineering technologies varies by region, creating uneven market access and potentially limiting the geographic scope of conservation projects.
The proposed biodiversity credit market remains theoretical, with uncertain market acceptance and regulatory support in federal sustainability funding. The absence of established valuation methodologies for biodiversity credits creates pricing uncertainty, while the long-term governmental commitment to conservation financing programs faces political volatility. These interconnected regulatory and revenue uncertainties may affect Colossal Biosciences' timeline for bringing de-extinct species to targeted ecosystems and achieving sustainable revenue streams to justify current valuations.
Summary
Colossal Biosciences is a synthetic biology and genetic engineering company developing de-extinction technology for projects targeting the woolly mammoth, thylacine, dodo, and dire wolf, with the company claiming it is the first to successfully de-extinct a species with the dire wolf. Although Colossal Biosciences’ stated mission is focused on ecosystem conservation and restoration, the company operates akin to the technology transfer approach of NASA, with a multi-faceted business model that combines technology spinoffs (Form Bio for computational life sciences and Breaking for plastic degradation), government partnerships for conservation services, and potential future biodiversity credits or ecotourism.
While the accelerating biodiversity crisis and expanding synthetic biology market create opportunities for Colossal Biosciences’ genomic and reproductive technologies, the company could face challenges successfully rewilding extinct species, navigating changing regulatory frameworks, and maintaining its social license for pursuing de-extinction and other gene editing applications. Colossal Biosciences will look to continue balancing its moonshot scientific and conservation goals with translating its technological developments into commercially viable revenue streams to justify its over $10.3 billion valuation as of November 2025, a figure that represents investor confidence in its future potential despite limited current revenue generation.